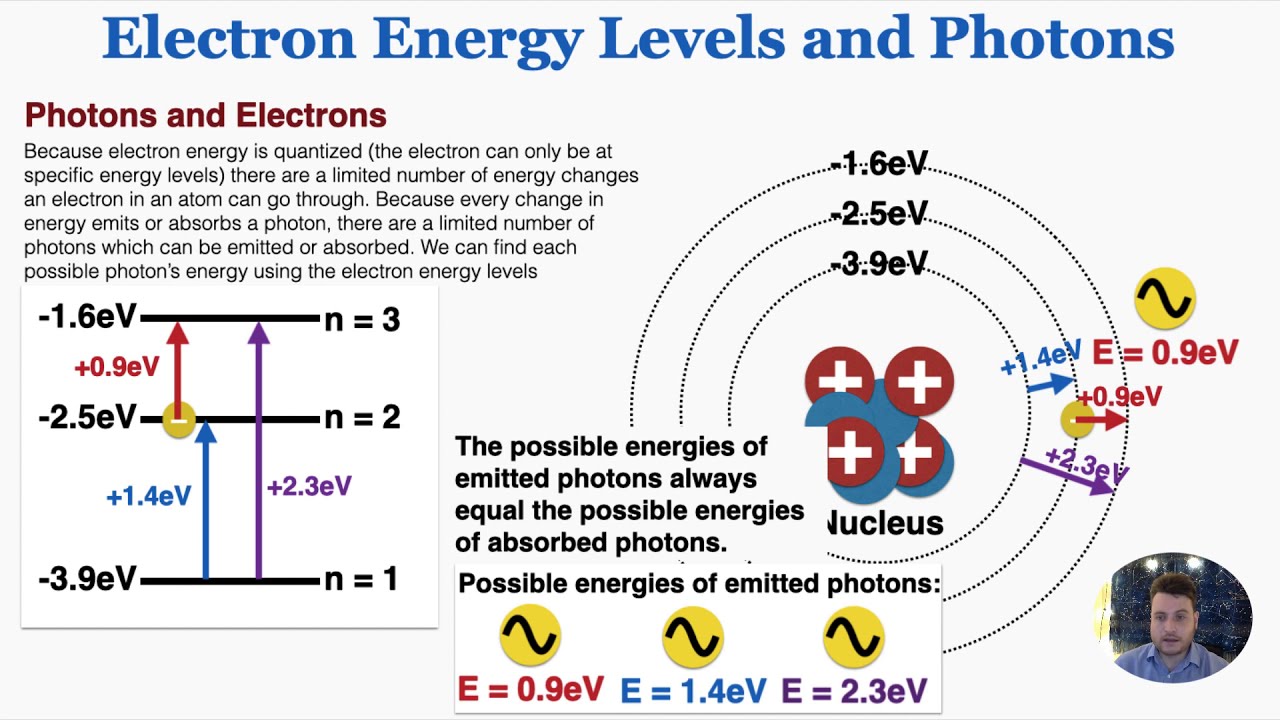
Energy Caused By Electrons
Calculate the kinetic energy and potential energy of an electron in the Bohr model hydrogen atom Electron photons physics
What factors can affect the energy of an electron inside an atom?
What are semiconductors? – materials science & engineering Electron orbit nth bohr hydrogen atom Electron quantum orbitals shell atomic levels electrons orbital nucleus probable
1. electron configuration
Hydrogen atom: january 2017Energy electron levels configuration electrons level atom nucleus each orbit lowest down Cathode simulation electrons puddingBohr model.
Energy hydrogen model bohr atom electron levels potential kinetic postulates bohrs physics chemical sumElectromagnetic radiation Electron energy levels of atomsEnergy electron kinetic potential atom hydrogen calculate orbit first topperlearning 4th pm jun answered expert.

Electron energy levels example
Absorption light lines electromagnetic radiation line hydrogen atoms spectra produce do absorbed production gif photons energies making astronomynotesEnergy photons quantization photoelectric effect quantum electrons chemistry quantized theory light libretexts high chem atoms structure chapter electronic full metal Energy electron example levelsHydrogen atom electron velocity supplemental significance.
Energy electricity electrical diagram flow electron thermal types called knowledge bank learn shows objects bulb use solar schoolsElectron energy atom shielding electrons affect Interaction of radiation with matterGif energy electron state states potential tiger excited ground ncssm structure.

Powerpoint presentation quantum numbers and atomic orbitals
Bohr quantum atom electron nucleus orbits niels electrons orbit orbital orbitals jumping britannica mechanics transition photon absorbing 1913 labeled emittedEmission photon electron hydrogen transitions bohr highest absorbs aamc fl4 Electronenergylevels.jpgLight & matter interaction.
Electrical energyMetals conduct semiconductors electrons atom electron valence atoms terrifyingly The quantization of energyElectron energy levels and photons.
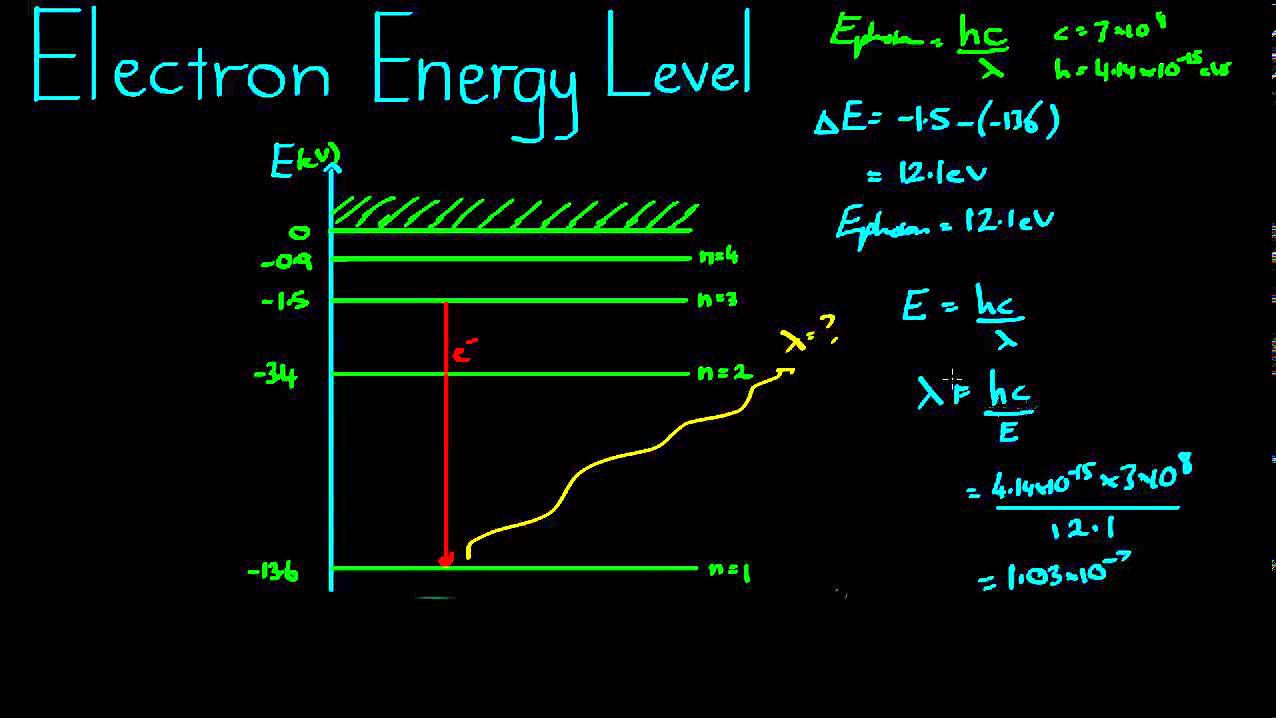
Interact astronomy student
Physics notes for high school: how energy of electrons is converted inInteraction matter radiation ionization charged particles electron interactions excitation atomic orbital bremsstrahlung resulting atoms less close figure radiologykey Energy electron levels atoms structure molecularWhat factors can affect the energy of an electron inside an atom?.
Energy of electron| nth bohr's orbit|hydrogen atom|formula .